Taking stock of Idebenone
By Peter Makai PhD and Christina Barckhausen PhD
Idebenone is a registered medicine for the disease Leber hereditary optic neuropathy (LHON), which is similar to ADOA. Idebenone is therefore one of the oldest and demonstrably safe medications to slow the progression of ADOA. ADOA patients receive this drug in some countries, such as Germany, through a so-called off-label procedure. But not in other countries such as the United Kingdom, the Netherlands or Sweden. Idebenone is often available there as a dietary supplement. One of the most frequently asked questions at the Cure ADOA Foundation is whether patients should take Idebenone.
In this article we will take stock of the research on Idebenone and hopefully this will help patients talk to their doctor if they have this question.
To do this, we need to look at how OPA1 mutations damage mitochondria. This is explained in Figure 1. Mitochondria contain two membranes, the inner and the outer mitochondrial membrane. The main function of OPA1 is the organization and renewal of the inner mitochondrial membrane, where, for example, energy for the cells is produced. The energy molecule ATP is produced in the elongated tubes in the mitochondria, by so-called mitochondrial energy-producing complexes. When OPA1 is defective, the inner mitochondrial membrane is “disordered” which drastically hinders ATP = energy production. In other words, it hinders cellular energy production.
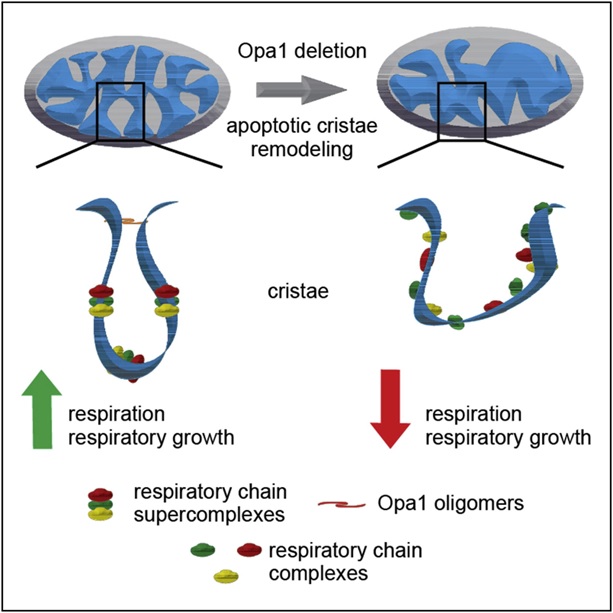
Figure 1. Disorganized membrane caused by lack of OPA1. With adjustments adapted from Cogliati (2013)
In addition to reduced energy production, a lack of OPA1 is accompanied by an excessive production of toxic molecules, so-called reactive oxygen species (ROS). Normally there is an adequate level of ROS, but if OPA1 is missing, there is too much ROS. This is shown in Figure 3.
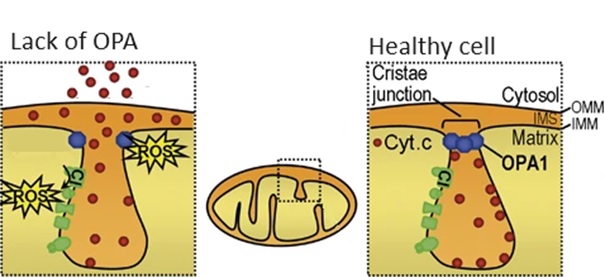
Figure 2 Regulation of ROS by OPA. Adapted from Ramonet (2013) with adjustments
In addition, the fusion of two different mitochondria with each other, a prerequisite for the proper functioning and maintenance of mitochondria, is disrupted (Figure 3). This is especially important when mitochondria are damaged. Much of the remaining function can be saved if the mitochondria fuse with a healthy mitochondria. If fusion is not possible due to the lack of OPA1, defective mitochondria accumulate. When a cell has many defective mitochondria, cell death occurs and in ADOA the RGCs die, leading to reduced vision. In essence, sufficient OPA1 protects against cell death.
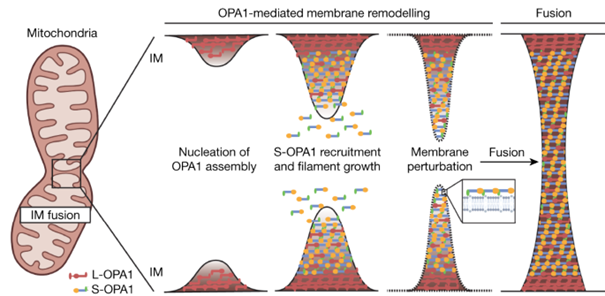
Figure 3 The role of OPA1 in the fusion of (damaged) mitochondria.
Other important functions of OPA1 include maintaining healthy levels of calcium ions in the cell and protecting mitochondrial DNA. The latter contains the building plan for mitochondria and is needed when something needs to be repaired (Leaners et al., 2020). All these processes are negatively affected when inner mitochondrial membrane renewal is disrupted by a lack of intact OPA1.
Another important aspect is the details of ATP production, shown in Figure 4. ATP is produced in the elongated tubes within the mitochondria, by mitochondrial energy-producing complexes. The energy-producing complex consists of successive complexes, complex 1-5, with 5 producing the energy molecule ATP. In the complexes, electrons are transferred from one complex to the next. An important molecule here is the coenzyme Q, (Q in figure 3), which links complex 1-2 with 3.

Figure 4 ATP production and the energy-producing complex. Andrieux (2021)
Idebenone
Although Idebenone was thought to play a role in directly enabling ATP production, recent research suggests that it mainly has other roles (Gueven et al, 2020). These are (1) transporting ATP building blocks to the mitochondria, (2) stabilizing the mitochondrial membrane, and (3) reducing ROS. Idebenone increases the levels of ROS-detoxifying enzymes, which eliminate excess ROS, thus protecting the mitochondria.
Idebenone was initially developed as a synthetic version of the coenzyme Q10/ (also called Ubiquinone) analogue with greater accessibility to all “regions” (compartments) of a cell. The natural coenzyme Coenzyme Q10 is known for its crucial role in energy production and protection against the above-mentioned toxic molecules called ROS. At the same time, natural coenzyme Q10 has the disadvantage that it does not reach its target in the cell, hence the need for a synthetic version. Although Idebenone shares structural features with the natural coenzyme Q10, its main mechanism of action differs from that of Q10 (Gueven et al. 2015, Gueven et al., 2020). Like Q10, Idebenone is likely able to support ATP = energy production (by directly transferring electrons to complex II and III of the energy production chain). Furthermore, unlike Q10, Idebenone can transfer energy equivalents (=ATP precursors) produced in the cell fluid to the mitochondria to increase ATP output (Gueven et al. 2015).
Furthermore, Gueven et al. 2020 state that the main protective mechanism of Idebenone is the reduction of toxic free radicals through a more effective mechanism than Q10 does: Idebenone not only reacts directly with free radicals and thereby scavenges them, as Q10 does, but also increases the levels of free radical neutralizing enzymes (imagine them as little machines). This detoxification protects sensitive cell components such as nuclear and mitochondrial DNA or lipids. In addition, the following indications require further investigation: Idebenone promotes “stem cell properties” of a cell (possibly interesting for RGC/optic nerve regeneration), promotes cell survival, increases the levels of energy production chain proteins, increases the number of mitochondria within a cell and reduces stress on the endoplasmic reticulum (another important cell organelle) (Gueven et al., 2020).
Medical evidence
The biology is quite complex, but the most important question is: does it really work? In Europe the drug is called Raxone and is produced by Chiesi. Raxone is approved as a drug for Leber's hereditary optic neuropathy, which has similarities to ADOA. There have been several studies to investigate whether Raxone has benefits for ADOA patients. Raxone has several additional molecules (excipients) that can help Idebenone achieve its purpose. Like all medications, it has side effects, which are described in the prescription. Here we focus only on the effect of Raxone on the eyes.
To understand these studies, it is necessary to look at the methods by which medical evidence is generated. The most common measurements are visual acuity and color vision.
Visual acuity is usually measured on a chart such as the one below (Figure 5). Eyesight is often expressed in decimals (0 total darkness to 1 perfect vision).

Figure 5. Snellen chart found in many doctor's offices.
Another way to measure visual acuity is the logMAR. This goes from (1 top row to 0) bottom row.
Figure 6 A graph of logMAR scores.
Color perception is also tested with a color test. Figure 7 below shows such a test.
Figure 7. Color vision test.
In addition, questionnaires are used and converted into 1-100 scores to assess the patient's quality of life, for example experience with daily activities, driving, etc.
The investigations
There are three studies on the effectiveness of Idebenone, the first (Barboni et a. 2013), the second (Romagnoli 2020) and the third (Valentin et al 2023).
Barboni gave idebenone to 7 patients and performed a measurement a year later. Vision is often expressed in decimals (0 total darkness to 1 perfect vision). (Barboni et a. 2013) showed that on average there was an improvement of 0,05 in the left eye and a difference of 0,09 in the right eye. There was also some improvement in color vision.
Romagnoli did a much larger study, with 87 patients, where 50 patients were treated with Idebenone and 37 were not. The patients were followed for 3-4 years. Comparing patients with untreated patients has the advantage of giving you an idea of what would have happened to the treated patients if they had not received Idebenone. Essentially, the researchers compared Idebenone to the natural history of the disease. This study used a different method to look at visual acuity, logMAR, which ranges from 1 (top row to 0 (bottom row). On average, (Romagnoli 2020) found a difference of 0,01 logMAR (about 0,5%) , and it was unlikely that this difference was due to chance. At the same time, the researchers found that more patients in the Idebenone group appeared to be stabilized (or slightly improved) than in the group without Idebenone.
The third study, by Valentin, gave Idebenone to 16 patients and followed them for a year. LogMAR was also used here (Valentin et al 2023) and an improvement of 0,08 for the right eye and 0,06 for the left eye was found. Valentin and colleagues also looked at the visual field (which part of the eye sees well) and found a significant improvement after 9 months. There was also a significant improvement in quality of life, with patients able to do more remote activities and being better able to drive (Valentin et al 2023).
Overall, the medical evidence so far is mildly positive, but rather inconclusive, and more studies would be needed to provide definitive evidence of effectiveness. We also don't know anything about its long-term effectiveness, and this is important because if Idebenone is actually able to stabilize (or possibly slightly improve) vision over a number of years, that would be very useful indeed. On the other hand, the improvement may be temporary, followed by a relatively faster decline. We have been in contact with Chiesi several times and they have no plans to look at ADOA for this disease. Having clear evidence is necessary in many countries to reimburse the cost of a medicine (UK, Netherlands, Sweden, etc.). At the same time, Raxone can be prescribed in other health systems, such as Germany.
Self-medication
If a particular pharmaceutical is not available in a country, self-medication may be an option. Idebenone is also available as a dietary supplement in many countries. The Cure ADOA Foundation has asked an independent laboratory to test the nutritional supplement and compare it with Raxone. The amount of Idebenone appears to be the same. There may be differences in the way the molecule reaches the target, it is possible that Raxone has a targeting system that helps to reach the target, which may not be present in the dietary supplement. But due to the uncertainty about how exactly Idebenone works, the (limited) benefits of Idebenone may also occur without the delivery system in Raxone to achieve the goal. In summary, although there appear to be no overall risks, this is not a panacea and ADOA remains an unresolved condition.
References
Andrieux, Pauline, et al. “Mitochondria as a cellular hub in infection and inflammation.” International journal of molecular sciences 22.21 (2021): 11338
Barboni, Piero, et al. “Idebenone treatment in patients with OPA1-mutant dominant optic atrophy.” Brain 136.2 (2013): e231-e231.
Cogliati, Sara, et al. “Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency.” Cell 155.1 (2013): 160-171
Gueven, Nuri, Krystel Woolley, and Jason Smith. “Border between natural product and drug: comparison of the related benzoquinones idebenone and coenzyme Q10.” Redox biology 4 (2015): 289-295.
Gueven, Nuri, et al. “Idebenone: When an antioxidant is not an antioxidant.” Redox biology 38 (2021): 101812.
Ramonet, D., et al. “Optic atrophy 1 mediates mitochondria remodeling and dopaminergic neurodegeneration linked to complex I deficiency.” Cell Death & Differentiation 20.1 (2013): 77-85
Romagnoli, Martina, et al. “Idebenone increases chance of stabilization/recovery of visual acuity in OPA1‐dominant optic atrophy.” Annals of clinical and translational neurology 7.4 (2020): 590-594.
Valentin, Katharina, et al. “Idebenone Treatment in Patients with OPA1-Dominant Optic Atrophy: A Prospective Phase 2 Trial.” Neuro-Ophthalmology 47.5-6 (2023): 237-247.
von der Malsburg, Alexander, et al. “Structural mechanism of mitochondrial membrane remodeling by human OPA1.” Nature 620.7976 (2023): 1101-1108



